Abstract
Introduction: The 2022 ICC classification identifies mutations in nine genes that classify AML with myelodysplasia-related gene mutations (AML-MRG). AML-MRG is associated with an unfavourable prognosis according to the 2022 ELN classification. However, it is unknown whether the clonal hierarchy of those mutations impacts prognosis.
Methods: 485 adult newly-diagnosed AML patients (median age 54) with available genetic and follow-up data were included. Mutations present at diagnosis were identified by Illumina myeloid panel sequencing covering 56 AML-associated genes. Patients received standard induction and consolidation chemotherapy or underwent allogeneic hematopoietic cell transplantation (alloHCT). MRG mutations were classified as subclonal if their variant allele frequency (VAF) was ≥ 10% below the mutation with the highest VAF of other co-occuring mutations. Otherwise, MRG mutations were considered part of the dominant clone.
Results: 177 (36.5%) patients carried at least one of the nine MRG mutations ASXL1, BCOR, EZH2, RUNX1, SF3B1, SRSF2, STAG2, U2AF1 and ZRSR2. The MRG mutation was found in the dominant clone in 134 (75.7%) and in a subclone in 43 patients (24.3%). BCOR, RUNX1, SRSF2, STAG2 and U2AF1 were more likely found in the dominant clone, whereas ASXL1, EZH2, SF3B1 and ZRSR2 were more often found in a subclone.
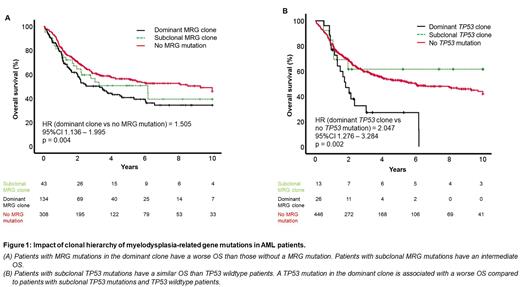
Patients with MRG mutations in the dominant as well as a subclone were significantly older than patients without MRG mutations (p < 0.001 and p = 0.03, respectively). Patients with MRG mutations in the dominant or a subclone and patients without MRG mutations achieved CR/CRi in 80.6%, 83.7% and 87.3%, respectively (p = n.s.). In line with the ELN classification, patients with MRG mutations in the dominant clone had a worse OS than patients without MRG mutations (p = 0.004, HR = 1.505, 95%CI 1.136-1.995); however, patients with MRG mutations in a subclone had a similar OS compared to patients with MRG mutations in the dominant clone and to patients without MRG mutations (Figure 1A).
TP53 mutations with ≥10% VAF are defined as AML with mutated TP53 by ICC 2022. 39 (8%) patients carried a TP53 mutation. One third of the cases had a subclonal TP53 mutation. Median TP53 VAF was 39.6% and 15.5% in patients with dominant or subclonal TP53 mutation (p < 0.001). OS was significantly worse in patients with dominant TP53 mutation compared to TP53 wildtype patients (p = 0.002, HR = 2.047, 95%CI 1.276-3.284), whereas OS was similar in patients with subclonal TP53 mutation compared to TP53 wildtype patients (Figure 1B).
Conclusion: In our study, 76% of MRG mutations occur in the dominant clone in newly diagnosed AML patients. Dominant MRG mutations have a worse prognosis compared to patients without MRG mutations, while prognosis of subclonal MRG mutations should be evaluated in larger cohorts. AML patients with subclonal TP53 mutation have a similar outcome to patients without TP53 mutation.
Disclosures
Lübbert:Cheplapharm: Other: Study drug; Syros: Consultancy; Otsuka: Consultancy; Janssen: Research Funding; Astex: Honoraria; Abbvie: Honoraria. Gaidzik:Abbvie: Honoraria, Speakers Bureau; Janssen: Speakers Bureau; Jazz Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; Pfizer: Speakers Bureau. Döhner:AbbVie: Honoraria, Membership on an entity's Board of Directors or advisory committees; Jazz: Honoraria, Membership on an entity's Board of Directors or advisory committees; BMS/Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Agios: Research Funding; Astellas: Research Funding; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Kronos: Research Funding. Döhner:Kronos Bio, Inc.: Research Funding; AbbVie Inc.: Consultancy, Honoraria, Research Funding; AstraZeneca: Honoraria; Berlin-Chemie: Consultancy, Honoraria; Brystol Myers Squibb: Consultancy, Honoraria, Research Funding; Jazz Pharmaceuticals: Consultancy, Honoraria, Research Funding; Novartis: Consultancy, Honoraria, Research Funding; Daiichi Sankyo Co, LTD: Consultancy, Honoraria; Gilead Sciences, Inc.: Consultancy, Honoraria; Janssen Pharmaceuticals: Consultancy, Honoraria; Servier: Consultancy, Honoraria; Syndax Pharmaceuticals Inc.: Consultancy, Honoraria; Pfizer Inc.: Research Funding; Amgen Inc.: Consultancy, Honoraria, Research Funding; Agios Pharmaceuticals: Consultancy, Honoraria, Research Funding; Astellas Pharma Inc.: Consultancy, Honoraria, Research Funding. Thol:AbbVie: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; BMS: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees. Heuser:Jazz Pharmaceuticals: Consultancy, Honoraria, Research Funding; Janssen: Honoraria; Novartis: Consultancy, Honoraria, Research Funding; Takeda: Honoraria; Agios: Consultancy, Research Funding; BMS: Consultancy; Daiichi Sankyo: Consultancy, Research Funding; Glycostem: Consultancy, Research Funding; Kura Oncology: Consultancy; Pfizer: Consultancy, Research Funding; PinotBio: Consultancy, Research Funding; Roche: Consultancy, Research Funding; Tolremo: Consultancy; Abbvie: Consultancy, Honoraria, Research Funding; Eurocept: Honoraria; Astellas: Research Funding; Bayer Pharma AG: Research Funding; BergenBio: Research Funding; Loxo Oncology: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal